Hepatology
PharmaNest’s FibroNest™ and FibroMAP™ platforms have been deployed across multiple liver disease etiologies, including Hepatitis B & C, MAFLD, pre-cirrhotic MASH, cirrhosis, PBC, and acute alcoholic liver disease, within diverse therapeutic and surgical contexts. The company is currently conducting three outcome studies involving over 3,500 patients with MASH to confirm the prognostic value of its digital fibrosis biomarkers.
Antifibrotic Compound MID-ThroUGHPUT Screening
FibroNest™ enables sensitive, quantitative evaluation of antifibrotic compounds by measuring single-fiber remodeling with high precision. In the absence of conventional staging, it detects early therapeutic effects, supports dose–response ranking, and ensures reproducible, cross-model comparisons—accelerating candidate selection in novel biological systems.
“Digital pathology with artificial intelligence analysis provides insight to the efficacy of antifibrotic compounds in human 3D MASH model.” Scientific Reports 2024; 14:5885.
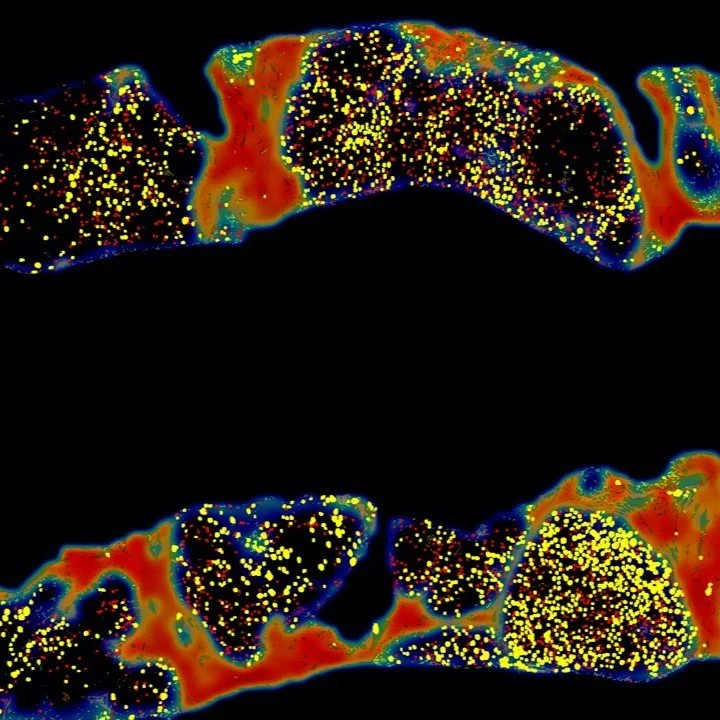
IND-Enabling Studies Fibrotic Genetically-driven models
FibroNest™ provides quantitative, fibrosis phenotyping in immuno-fibrotic and genetically driven models, delivering high-resolution endpoints for IND-enabling studies. It captures subtle structural changes in collagen architecture, enabling early detection of therapeutic effects, improved dose effect quantification, and mechanistic validation across immune, genetic, and toxin-induced fibrosis models
“Mannose reduces fructose metabolism and reverses MASH in human liver slices and murine models in vivo.” Hong et al. Hepatology Communications 2025; 9(4):e0671.
intra-stage Quantification of Therapeutic response
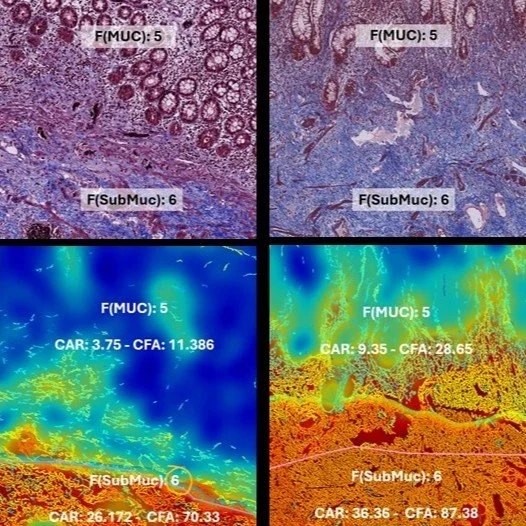
FibroNest™ enables precise intra-stage quantification of therapeutic response, capturing fibrosis regression or stabilization even within the same histological stage. By providing continuous digital scores, it distinguishes subtle matrix remodeling missed by categorical grading, supporting early efficacy detection and refined endpoint interpretation in clinical trials
Aramchol improves hepatic fibrosis in MASH: Results of multimodality assessment using both conventional and digital pathology. Ratziu et Al, Hepatology, 2024 (79), issue 4, April 7
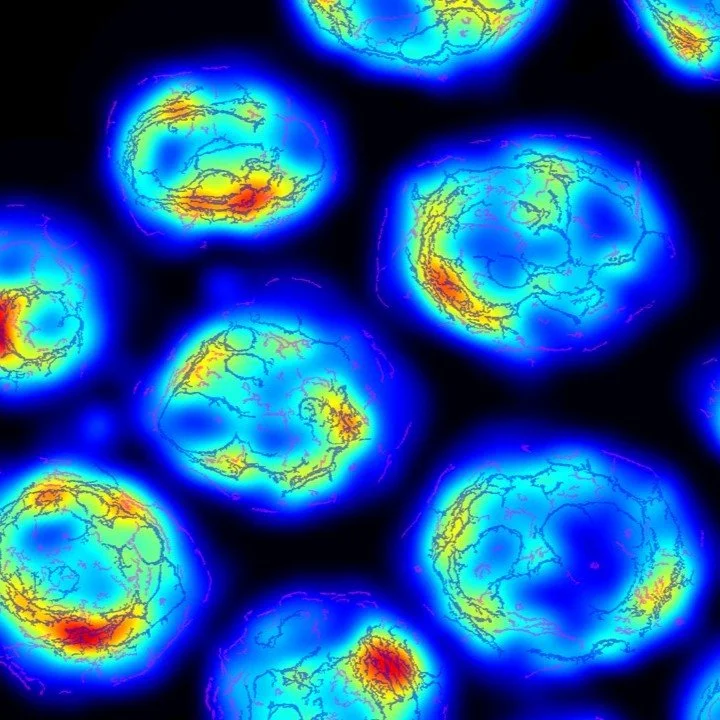
Automated Fibrosis Phenotyping Reveals HCC Fibrotypes
Automated digital fibrosis phenotyping of liver tissue from transplant patients with and without hepatocellular carcinoma uncovered unique collagen architecture patterns linked to carcinogenic progression. FibroNest™-based phenotypic analysis can differentiate fibrotic micro environments in NAFLD, providing histological biomarkers that enhance early detection and risk stratification cancer development.
Automated fibrosis phenotyping of liver tissue from non-tumor lesions of patients with and without hepatocellular carcinoma after liver transplantation. Nakamura et Al. Hepatol Int. 2022;16(3):555-561.
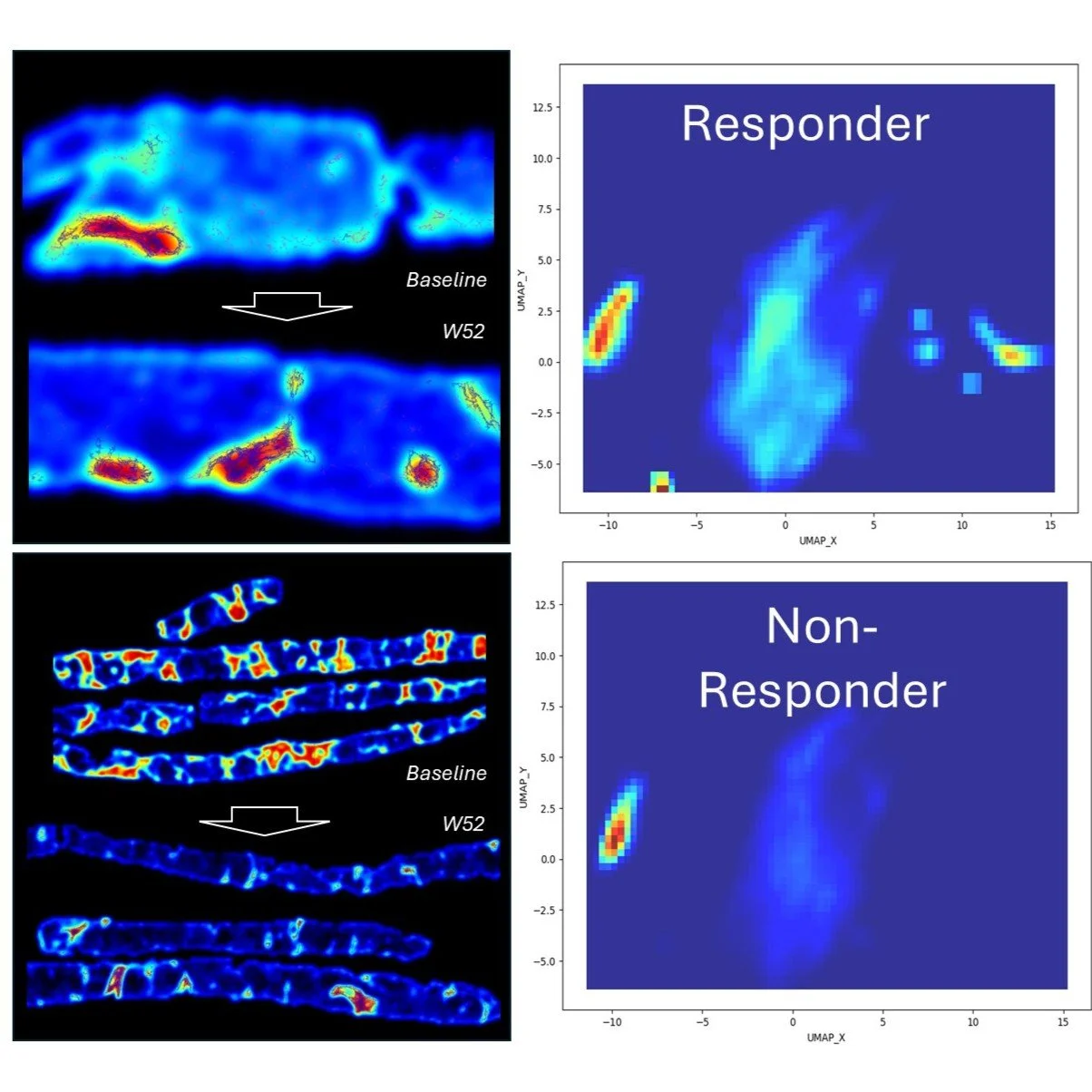
Spatial Computational Histology Predicts Antifibrotic Response
In the Phase 2b FASCINATE-2 MASH study, FibroMAP™ analyzed 246 liver biopsies using single-fiber spatial phenotyping to stratify denifanstat responders. By clustering over 300 quantitative fibrosis traits, FibroMAP™ achieved strong predictive accuracy (AUROC = 0.77–0.81), identifying unique fibrotic phenotypes linked to treatment response. This demonstrates FibroMAP’s power to uncover spatial biomarkers of therapeutic efficacy.
Spatial Computational Histology Stratified Denifanstat Fibrosis Responders in the Phase 2b FASCINATE-2 MASH Study. Ratziu et al. AASLD 2025 - Hep. August 2025, (82), S1 (S787) -
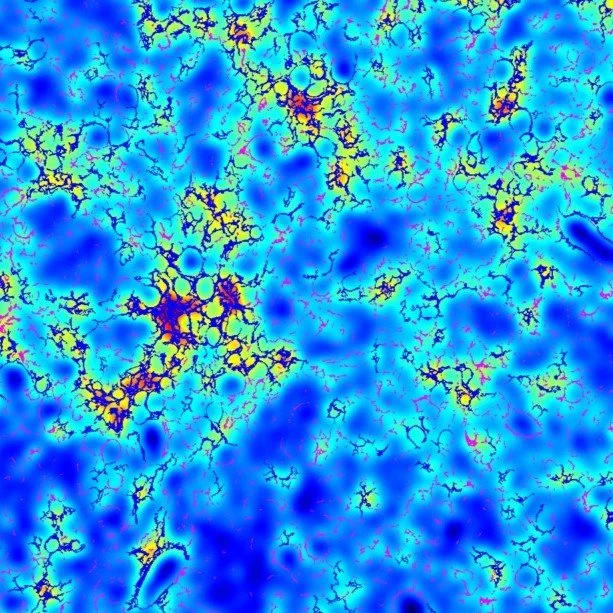
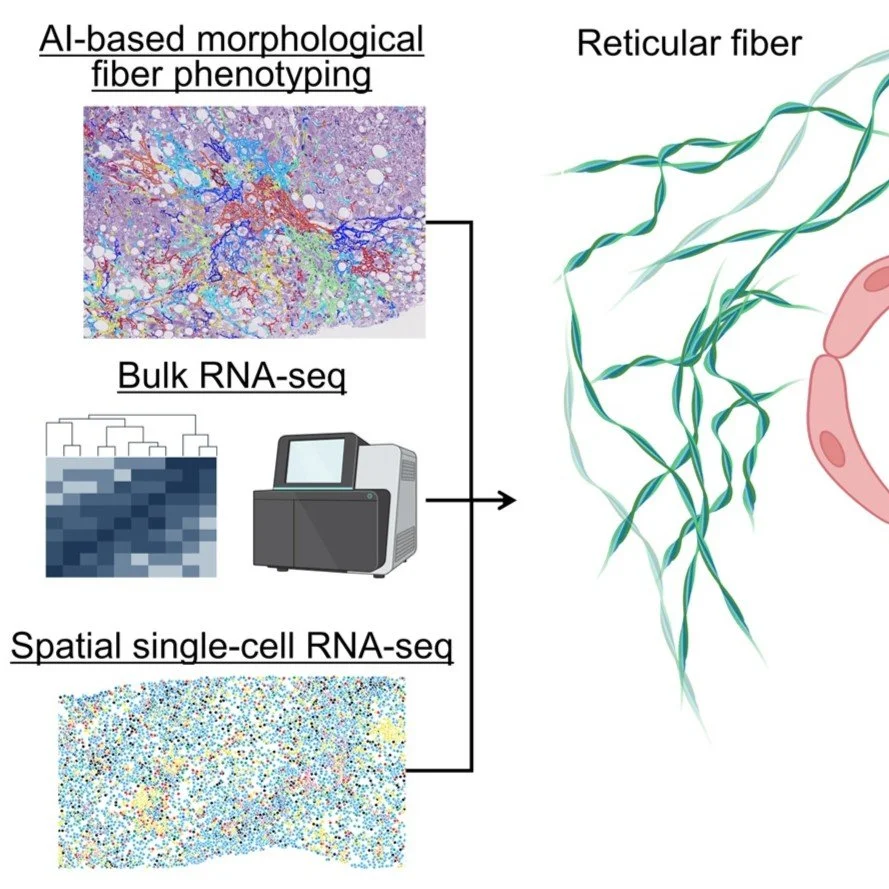
SINGLE-FIBER and SINGLE-CELL SPATIAL FUSION: NOVEL FIBROTYPES OF hcc
FibroNest™ and FibroMAP™ enable the integration of single-fiber digital histology with single-cell and spatial transcriptomic data, linking tissue architecture to molecular signatures of disease. This spatial fusion reveals how specific collagen fiber phenotypes correspond to transcriptional programs in hepatic stellate cells and inflammatory pathways, advancing precision medicine through structure–function mapping.
“AI-based phenotyping of hepatic fiber morphology to inform molecular alterations in metabolic dysfunction–associated steatotic liver disease.” Fujiwara et al. Hepatology 2025 Apr 22:10.1097